ARTICLE
Medical case reports published in PubMed-indexed Indian journals in 2015: Adherence to 2013 CARE guidelines
Renju Ravi, Alhad Mulkalwar, Urmila M Thatte, Nithya J Gogtay
Published online: April 23, 2018
DOI: https://doi.org/10.20529/IJME.2018.036
Abstract
In 2013, an independent group of researchers developed the CARE guidelines, a checklist to standardise reporting of case reports. This study assesses adherence to CARE guidelines among PubMed-indexed Indian medical journals in 2015 and the extent of endorsement of these guidelines by the journals. Case reports published in 2015 in journals indexed by PubMed, belonging to the medical stream, currently active, and that had an impact factor were included for analysis. Case series and journals that were published from India but for another country were excluded. Total adherence score and classification of adherence as “excellent”, “very good”, “good”, and “poor” as also adherence to individual components of the checklist were the outcome measures. A total of 162 journals were identified by the search strategy, of which 36 satisfied the selection criteria. In these 36 journals, 1178 case reports were published. We tested the association between the type of journal and impact factor with adherence by using the chi-squared test and generated crude odds ratios. All analyses were done at 5% significance. Based on the total percent score, no case report had excellent adherence, and 19% had good, 70.7% average, and 10% poor adherence, respectively. Among the sub-items, the best adherence was seen in the clinical findings [97.9%], followed by keywords [88.5%], and introduction [71.5%]. The items with extremely poor adherence were patient perspective [0%], informed consent [2.8%], and timeline [4.6%]. Journals with an impact factor of more than 1 had better adherence, relative to those with an impact factor lower than 1. Only one journal’s website mentioned the CARE guidelines. Greater awareness needs to be created among authors, peer reviewers, and editors about using these guidelines. As informed consent is a metric of autonomy, all stakeholders must ensure its reporting.
Background
A case report is defined as the scientific documentation of a single clinical observation (1). The use of case reports has been, and will always remain, an important tool for advancing clinical knowledge as also for teaching and training purposes. The inherent strength of the case report lies in its ability to weave together two narratives—one of the patient and the other of the treating physician (2). Hence, presenting case reports appropriately in medical literature is important.
In 2013, for the first time, standards for the reporting of case reports called the CARE (CAse REport) guidelines were developed by an independent international group of experts (3). The idea behind their development was to improve completeness and transparency of publication so that well-written case reports could subsequently inform provision of healthcare and provide early signals of safety and benefit (4). At the time of this study, unlike with the CONSORT guidelines, only a limited number of journals internationally had endorsed these guidelines (3). Against this backdrop, we carried out the present study with the primary objective of assessing adherence of case reports to CARE guidelines among PubMed-indexed Indian medical journals in one year. A secondary objective was to assess the extent of endorsement of these guidelines by the journals.
Methods
Ethics
We submitted the study protocol to the institutional ethics committee of Seth GS Medical College and KEM Hospital, which deemed it exempt from review on September 17, 2016 (EC/OA-146/2016) as the data was available in the public domain.
Selection criteria and study sample
We conducted the study for the year 2015 as this would be two years after the guidelines were introduced. Inclusions were: 1) case reports published in that one year; 2) Indian journals from medical specialities indexed by PubMed; 3) journals currently active and with an impact factor. Case series and those journals that were published from India but for another country were excluded.
Search strategy
We searched the Medline/PubMed database using the search strategy {India* AND medicine [MeSH] NOT “Specialities, Surgical” [MeSH], India [publisher] AND health occupations [MeSH], India [pl] NOT India [publisher] AND health occupations [MeSH]}.
Use of the checklist, scoring, and calculation of adherence
We used the 13-item CARE checklist (5); individual components were allocated weighted scores, and a total score per report was calculated. We then converted the total score into a percent total score. Based on percent score ranges, the case reports were classified as having excellent (100–90%), good (89–70%), average (69–50%), or poor (49% or lower) adherence. Adherence for the sub-items of the CARE guidelines was similarly calculated. All of these were done through consensus in a series of meetings among the authors. Journals identified via the search were classified as general medical, speciality, or super-speciality journals and also divided into those with an impact factor more than 1 or less than 1.
Outcome measures
The proportions of case reports in each adherence category (“excellent”, “very good”, “good”, and “poor”) were analysed. Likewise, proportions of case reports that adhered to each sub-item of the CARE guidelines were also analysed.
Statistical analysis
We applied both descriptive and inferential statistics. The total score was expressed as mean and standard deviation (SD), while the number of journals in each category was expressed as proportions. The association of the type of journal and impact factor with adherence was assessed using the chi-squared test and crude odds ratio (COR) with 95% confidence intervals (CIs) generated. All analyses were done using Microsoft Excel, and a probability or “p” value of less than 5% was considered significant.
Post-study search
At the point of writing the paper, we looked at all the journal websites once again to see if they endorsed the CARE guidelines.
Results
Demographics
A total of 162 journals were identified by the search strategy. Of these, 126 (77.8%) were excluded for the following reasons: surgical or non-medical journals (37), not currently indexed (33), not currently active (24), published from India but for another country (20), and did not publish case reports (12). Of the 36 remaining as the final sample, 6 were general medical, 21 were speciality, and 9 were super-speciality journals. Relooking by impact factor, 29 had an impact factor below one while the remaining 7 had an impact factor of more than 1. A total of 1178 case reports were published in these journals in 2015. Table 1 gives the breakup of numbers of case reports published in each of these journals.
| Table 1: Distribution of the case reports (n = 1178) across journals (n = 36) | ||
| Type of journal | Number of journals n | Case reports n (%) |
| By scope | ||
| General medical | 6 | 400 (33.9) |
| Speciality | 21 | 497 (42.2) |
| Super-speciality | 9 | 281 (23.9) |
| By impact factor | ||
| More than or equal to 1 | 7 | 147 (12.5) |
| Less than 1 | 29 | 1031 (87.5) |
Overall adherence of case reports
The overall percent adherence score (expressed as mean [SD]) for all journals was 61.2% [9.2]. It was seen that 10.3% case reports had poor adherence, 70.7% average, and 19% good adherence. No case report fell into the excellent adherence category.
Adherence of sub-items of the CARE checklist
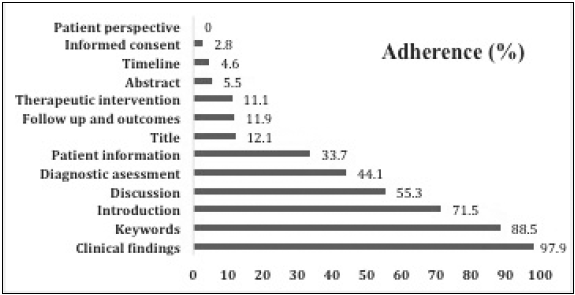
The best adherence was seen in the clinical findings (97.9%), followed by keywords (88.5%), and introduction (71.5%). The items with extremely inadequate/poor adherence were patient perspective (0%), informed consent (2.8%), and timeline 4.6%. Figure 1 depicts adherence of all case reports to individual components of the CARE checklist.
Association of adherence with impact factor
It was seen that case reports published in journals with an impact factor of more than 1 had better adherence scores relative to those published in journals with an impact factor less than 1 (COR 16.4 [9.3,17.8], p < 0.001).
Association of adherence with type of journal
Speciality journals had better overall adherence relative to general medical journals (64.81 [8.7] as against 58.15 [7.9], p < 0.001). Similarly, speciality medical journals also had better overall adherence relative to super-speciality journals (64.81 [8.7] as against 59.14 [9.4], p < 0.001).
Analysis of journal websites
At the point of the study, no journal from the study sample had endorsed CARE guidelines. A repeat search January 2018, done at the point of manuscript writing, showed that only one of the 36 journals endorsed the CARE guidelines. The need for informed consent prior to publication was mentioned by 26 (72%). Six of these journals needed consent only for publishing photographs.
Discussion
Of the 36 Indian medical journals we evaluated for adherence to CARE guidelines, almost three quarters had only “average” adherence. Only 3% case reports mentioned informed consent from the patient prior to publication, and only one journal mentioned the guidelines on its website.
Our findings are similar to observations made by other authors. Kaszkin-Bettag evaluated 150 case reports on metastasising basal carcinoma and found the quality to be uneven (6). The case reports did not mention the drugs used for chemotherapy. When mentioned, doses, duration, and chemotherapy cycles were missed. Kljakovic, in an audit of case reports published in general practice and general medical journals, similarly found that only 5% case reports reported informed consent (7). As both these audits preceded the publication of the CARE guidelines, one may expect an improvement in quality of reporting subsequent to their publication. Given that this has not happened, greater awareness needs to be created among authors, reviewers, and editors about the need to adhere to and endorse these guidelines.
Journals with an impact factor of more than 1 had better quality reporting. The journal impact factor (with all its fallacies) is used as a surrogate metric of quality of the journal (8). We are unable to explain the reason for this. However, the difference may or may not be a true difference as the number of journals with impact factor greater than 1 were only seven. What is more relevant is that with most Indian journals, editors usually work at middle- or senior-level positions at teaching institutes attached to university hospitals and editorial responsibility is added onto to an existing full-time job. This is the likely cause of inadequate attention paid to case reports and their publication, leading to low adherence as also a reflection of the inadequate quality of peer review.
Of all the findings, the poor reporting of informed consent is the most distressing. This may represent one of two things—consent was obtained but not reported or consent was not obtained—with the latter being more serious. It is also possible that consent reported by authors was not mentioned in the publication as a matter of editorial policy. Once editors adhere to CARE guidelines, this issue will be taken care of and there will be uniformity and transparency in reporting informed consent.
Unlike consent for participation in a clinical trial or undergoing a medical procedure, consent to publication of a case report has associated issues of possible disclosure of identity, and thus questions of privacy and confidentiality come up. Hence, taking the consent of the patient before publishing a case report is an ethical imperative (9). This is also now mandated by the recently released National Ethical Guidelines for Biomedical and Health Research Involving Human Participants released in 2017 by the Indian Council of Medical Research (ICMR) (10). Material within the case report may be identifiable (the face, for example) or non-identifiable when anonymised (X-rays, CT scans). Wherever the identity of the individual is at risk of being revealed, this must be explicitly stated in the consent form, and the patient must be counselled and must have consented (ICMR guidelines clause 10.8.2) (10). Ethical concerns about informed consent and confidentiality are best protected by the author(s), the journal editor, and the peer reviewers to whom the case report is submitted. Some journals published outside the country mandate that the case report be reviewed by the patient to permit editing or removal of any material that he/she would not want to disclose (11). They suggest creating consent forms, including additional consent for potentially identifiable information, and an opportunity for the patient/representative to actually review and approve the manuscript (12) and putting them up on their websites. While this may not always be possible in India, given the differential cultural context and literacy levels, informed consent is an imperative, with the patient receiving adequate explanation about the risks of revealing identity or disclosure of sensitive, private information where applicable. Among all the sub-items of the CARE checklist, this is probably the most important and must be given due attention by authors, peer reviewers, and editors.
Our study is limited by including only those articles published under the “case report” section of journals. We could have thus missed out case reports published under other sections such as “letter to the editor”, “images in medicine”, “case snippets”, or even “e-case reports”. It is also restricted to medical journals only, and that too from a single database (PubMed) for a single year. We did not include surgical case reports as there exists a different set of guidelines for them called the Surgical CAse REports (SCARE) guidelines (13). Also, the study was done only two years after the publication of the guidelines and this time period may have been inadequate for both dissemination and awareness.
In summary, an audit of Indian medical journals publishing case reports showed inadequate adherence to the 2013 CARE guidelines. This can be addressed by creating greater awareness about using these guidelines. As informed consent is a metric of autonomy, all three stakeholders—authors, editors, and peer reviewers—must ensure its reporting in all case reports.
Financial support: None declared
Conflicts of interest: None declared
References
- Carey JC. The importance of case reports in advancing scientific knowledge of rare diseases. Adv Exp Med Biol. 2010;686:77-86.
- Bayoumi AM, Kopplin PA. The storied case report. CMAJ. 2004 Sep 14;171:569-70.
- CARE Case Report Guidelines [Internet]. CARE Group (IMI LLC); 2018 [cited 2017 Dec 13]. Available from: http://www.care-statement.org.
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013 Oct 23;2013. pii:bcr2013201554.
- CARE Case Report Guidelines Checklist [Internet]. CARE Group (IMI LLC); 2018 [cited 2017 Dec 13]. Available from: http://www.care-statement.org/resources/checklist.
- Kaszkin-Bettag M, Hildebrandt W. Case reports on cancer therapies: the urgent need to improve the reporting quality. Glob Adv Health Med. 2012 May;1(2):8-10.
- Kljakovic M. Single cases in general practice and general medical journals. Aust Fam Physician. 2002 Jul;31(7):669-73.
- Bornmann L, Marx W. The journal Impact Factor and alternative metrics: A variety of bibliometric measures has been developed to supplant the Impact Factor to better assess the impact of individual research papers. EMBO Rep. 2016 Aug;17(8):1094-7.
- Barbour V, on behalf of COPE Council. Journals’ best Practices for ensuring consent for publishing medical case reports: guidance from COPE. Committee on Publication Ethics; 2016 Dec [cited 2018 Jan 4]. Available from: https://publicationethics.org/files/Best_Practices_for_Ensuring_Consent_for_Publishing_Medical_Case_Reports_guidance_from_COPE.pdf.
- Indian Council of Medical Research. National ethical guidelines for biomedical and health research involving human participants. New Delhi: Indian Council of Medical Research; 2017. Available from: http://www.icmr.nic.in/guidelines/ICMR_Ethical_Guidelines_2017.pdf.
- Shevell MI. The ethics of case reports. Paediatr Child Health. 2004 Feb;9(2):83-4.
- Yoshida A, Dowa Y, Murakami H, Kosugi S. Obtaining subjects’ consent to publish identifying personal information: current practices and identifying potential issues. BMC Med Ethics. 2013 Nov 25;14:47.
- Agha RA, Fowler AJ, Saetta A, Barai I, Rajmohan S, Orgill DP, for the SCARE Group. The SCARE Statement: consensus-based surgical case report guidelines. Int J Surg. 2016;34:180-6.
